Date: 22/03/2023


- Get your equipment
- Take a petri dish
- Take a weigh boat and place it on the center of your petri dish
- measure 3ml of acid using a measuring cylinder
- chop a small piece of moist apple and place it
- take a small piece of red cabbage and place it beside the apple but not to the point where they’re touching eachother
- Take a universal indicator and put enough drops that is small
- Take some moist calcium carbonate (to moisten the calcium carbonate is to drop some water but not to the point where it’s overflowing
- Take a bromothymol blue indicator and put it beside the universal indicator (make sure non of those touch eachother)
- Make sure your layout is like this first before doing anything (the petal is actually cabbage dm)

11. Once the layout is organised and ready, add a spatula of sodium sulfite in the weigh boat with the acid and quickly close the lid
(when the acid starts acting up you might feel a strong hit of smell that will hurt your nose)
Observe the reactions when it evaporates
Observation
Our bromothymol blue had changed into the colour orange
Universal indicator changed to red
Our moist calcium carbonate had broken down and changed colour
The cabbage had turned green and moldy ish
The apple may be different to ours (due to our size) but it’s supposed to decay a bit
The science
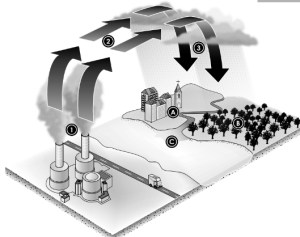
This indicates the reactions of what will happen to living things if we had acid rain happening
when Fossil fuels and natural gasses are burned, the carbon dioxide and other gases go into the air. The molecules dissolve in the water in the clouds and make the rain more acidic then before
Hi Stephanie, I like how you explicitly displayed your learning in this o ne, clearly explaining the steps and understanding the science in your learning.